Synthesis and adhesion control of glucose-based bioadhesive via strain-promoted azide–alkyne cycloaddition†
Abstract
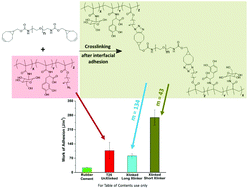
A glucose-based bioadhesive, poly(2-methacrylamido glucopyranose-co-N-methacryloyl-3,4-dihydroxyl-L-phenylalanine-co-8-azidooctyl methacrylate) [poly(MG-co-MDOPA-co-AOM)], has been synthesized by thermally-initiated free radical polymerization. The new bioadhesive is composed of three modules: a hydrophilic glycopolymer segment, a mussel-inspired catechol segment, and a crosslinking azide segment. Poly(ethylene glycol) (PEG)-based crosslinker, (1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-ylmethyl PEG (BCN-PEG) was synthesized separately. Bulk adhesion properties of the terpolymer were enhanced by covalent bond forming crosslinking via strain-promoted azide–alkyne cycloaddition (SPAAC). The occurrence of SPAAC was confirmed by 1H NMR and FT-IR. After moistening the adhesive and the crosslinker, BCN-PEG with water, adhesion properties were examined by a lap shear strength test on porcine skins. The control of adhesion was studied under various crosslinker concentrations, crosslinking durations, and crosslinker lengths. Even without crosslinking, the new terpolymer adhesive demonstrated 20-fold higher adhesion strength (115 kPa) compared to a commercial rubber cement (5.8 kPa). The most significant factor to control for adhesion was crosslinker length. BCN-PEG was prepared with 134 and 43 repeating units of PEG. Crosslinking with the long crosslinker, BCN-PEG (PEG repeating units: 134), did not enhance adhesion strength meaningfully. However, crosslinking the short crosslinker BCN-PEG (repeating units: 43) showed significant improvement in work of adhesion (150% higher than uncrosslinked). The overall revealed features of strong adhesion on biological surfaces, structural similarity to natural carbohydrate, water compatibility, controllability of adhesion strength, and non-toxic adhesion enhancement principle via SPAAC crosslinking, suggest that the new glucose-based bioadhesive can be successfully used for biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...