Promotion of micelle stability via a cyclic hydrophilic moiety†
Abstract
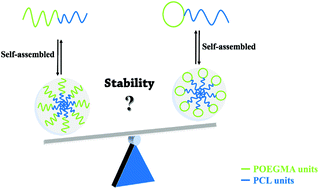
A novel strategy to promote micelle stability was reported by altering the topological structure of polymer species. Specifically, a cyclic hydrophilic moiety offers greater stability for the self-assembled micelles than a linear analogue. This study thus provides an alternative to enhance micelle stability for drug delivery.


 Please wait while we load your content...
Please wait while we load your content...