Radiant star nanoparticle prodrugs for the treatment of intracellular alveolar infections†
Abstract
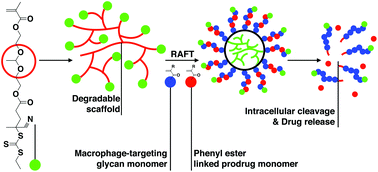
Radiant star nanoparticle (RSN) prodrugs were synthesized in a two-step process by first homopolymerizing RAFT transmers followed by copolymerization from the hyperbranched polymer core. Two trithiocarbonate-based transmers were synthesized containing either alkyl ester or acetal groups linking the polymerizable methacrylate group to the chain transfer agent (CTA). RAFT polymerization from the homopolymerized transmer cores yielded RSNs with linear polymer chains connected to hyperbranched cores. Hydrolysis studies conducted over a period of 30 days at 37 °C in acetate buffer showed that RSNs prepared from alkyl ester linked cores remained stable while acetal linked cores exhibited a progressive degradation into linear polymers over the same period. Macrophage targeting RSN prodrugs containing the antibiotic ciprofloxacin and receptor-targeting mannose residues were synthesized directly via RAFT polymerization of the prodrug and mannose monomers. Hydrolysis studies conducted in human serum showed that the RSNs released the covalently linked ciprofloxacin significantly faster than diblock copolymer micelles but moderately slower than soluble copolymers with comparable compositions. Flow cytometry showed substantially higher macrophage binding by the mannose-targeted RSNs while in vivo biocompatibility experiments showed no differences relative to phosphate buffer treated negative controls.


 Please wait while we load your content...
Please wait while we load your content...
