Synthesis and peptide functionalization of hyperbranched poly(arylene oxindole) towards versatile biomaterials†
Abstract
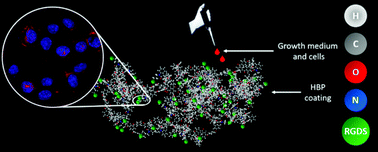
Hyperbranched polymers (HBPs) are dendritic macromolecules with a three-dimensional globular nanostructure. Due to their unique properties, HBPs are interesting for biomedical applications such as drug/protein delivery and tissue engineering, and functionalized HBPs can be tailored to exhibit specific biological responses. Superelectrophilic arylation of isatin is one strategy to synthesize hydrophobic hyperbranched poly(arylene oxindole)s from A2 and B3 monomers in one step. Herein, an azide derivative of this HBP, which allows postgrafting with a cell adhesive RGD peptide derivative via copper assisted azide–alkyne cycloaddition (CuAAC), is prepared. The resulting materials are shown to be cytocompatible and support cell attachment and proliferation; moreover, the amount of postgrafted peptide can be controlled. Overall, the demonstrated postgrafting strategy is compatible with different biomolecules, and the functionalized HBPs show high potential as biomaterials.


 Please wait while we load your content...
Please wait while we load your content...