Mild halogenation of polyolefins using an N-haloamide reagent†
Abstract
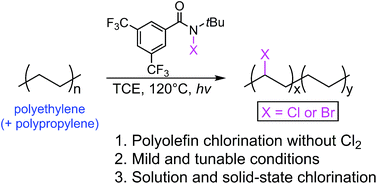
Chlorinated polyolefins remain highly valued commodity polymers owing to their excellent physicochemical properties. The chief synthetic route to this variety of polymer involves the chlorination of polyolefin materials using chlorine gas, an extremely toxic and heavily monitored chemical. We hereby report the application of an N-chloroamide compound in the chlorination of both polyethylene (PE) and polypropylene (PP). An N-bromoamide reagent was also synthesized and used in the bromination of PE samples in an analogous fashion. Polyolefin halogenation reactions, including chlorination and bromination, were performed in solution with various feed ratios and the products were characterized by multi-instrumental analysis including proton nuclear magnetic resonance, size exclusion chromatography, differential scanning calorimetry, Fourier transform-infrared spectroscopy and thermogravimetric analysis. A peak halogen content of 32.7 and 31.9 wt% was determined for chlorine and bromine, respectively. Moreover, a high-density PE film was additionally chlorinated and the result was verified by X-ray photoelectron spectroscopy analysis. The new synthetic methodology has been confirmed to be a tunable and convenient alternative to the use of chlorine gas for the production of chlorinated polyethylene.


 Please wait while we load your content...
Please wait while we load your content...