Cationic half-sandwich rare-earth metal alkyl species catalyzed polymerization and copolymerization of aryl isocyanides possessing polar, bulky, or chiral substituents†
Abstract
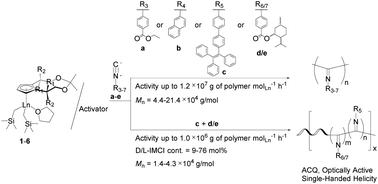
Two types of half-sandwich rare-earth metal dialkyl complexes (Cp)Ln(CH2SiMe3)2(THF) 1–6 (1: Cp = (3aR,4R,8R,8aR)-cyclopentadienyl ligand (Cpx*′), Ln = Sc; 2: Cp = Cpx*′, Ln = Y; 3: Cp = Cpx*′, Ln = Lu; 4: Cp = (3aR,8aS)-cyclopentadienyl ligand (Cpx*′′), Ln = Sc; 5: Cp = Cpx*′′, Ln = Y; 6: Cp = Cpx*′′, Ln = Lu) have been synthesized and structurally characterized by X-ray diffraction. The reaction of these half-sandwich rare-earth metal dialkyl complexes with almost one equivalent of activator (borate or borane) quantitatively afforded the cationic half-sandwich rare-earth metal alkyl species, which are the first example of rare-earth metal catalysts for the polymerization of five functional aryl isocyanides containing polar ester [4-ethoxycarbonyl phenyl isocyanide (EPI)], bulky naphthyl [2-naphthyl isocyanide (NI)] or tetraphenylethylene [4-isocyano-4′-(1,2,2-triphenylvinyl)-1,1′-biphenyl (ITPB)], or chiral ester [(1S,2R,5S)-2-isopropyl-5-methylcyclohexyl 4-isocyanobenzoate (D-IMCI) and (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 4-isocyanobenzoate (L-IMCI)] substituents with extremely high activities (up to 107 g of polymer molLn−1 h−1) under mild conditions. The resulting functional polyisocyanides have high molecular weights (Mn = 4.4–21.4 × 104 g mol−1) as well as broad molecular weight distributions (Mw/Mn = 3.20–6.82) and exhibit good solubility, aggregation-caused quenching (ACQ) nature, or single-handed helical conformation. Moreover, the complex 1/activator binary system can also promote the helix-sense-selective copolymerization of chiral D-IMCI or L-IMCI with achiral ITPB at high activities (up to 106 g of polymer molLn−1 h−1), affording new random poly(D/L-IMCI-co-ITPB)s with D/L-IMCI contents in the range of 9–76 mol% and different degrees of single-handed helical conformations. In contrast with the AIE nature of the ITPB monomer, these copolymers containing ITPB units exhibit ACQ nature similar to poly(ITPB). A plausible coordination–insertion mechanism is proposed based on ESI-MS spectroscopy, providing insight into the initiation and termination polymerization process of aryl isocyanides catalyzed by rare-earth metal catalysts.


 Please wait while we load your content...
Please wait while we load your content...