Room temperature multicomponent polymerizations of alkynes, sulfonyl azides, and N-protected isatins toward oxindole-containing poly(N-acylsulfonamide)s†
Abstract
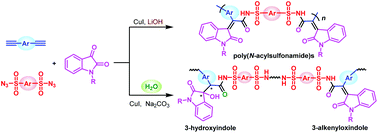
The development of a new polymerization methodology affords polymer materials with new structures and functionalities. Multicomponent polymerizations (MCPs) as a facile tool for preparing multifunctional polymers with complicated structures have attracted increasing attention from polymer scientists, owing to their high efficiency, high atom economy, simple procedure, structural diversity, and environmental benefit. In this work, a series of efficient one-pot multicomponent polymerizations of diynes, disulfonyl azides, and N-protected isatins are developed to afford oxindole-containing poly(N-acylsulfonamide)s with advanced properties. After optimization of the polymerization conditions, the MCP can proceed smoothly at room temperature or 30 °C in dichloromethane/t-BuOH with CuI as the catalyst and LiOH as the base, generating poly(N-acylsulfonamide)s with high molecular weights of up to 30 600 g mol−1 in excellent yields of up to 98%. This MCP enjoys general applicability of a series of electron-rich or electron-deficient alkynes and alkyl group or aromatic group-substituted isatins, generating six poly(N-acylsulfonamide)s from different combination of monomers, and nitrogen gas as the only byproduct, demonstrating high atom economy and environmental benefit. The obtained poly(N-acylsulfonamide)s can be dissolved in alcohol or alcohol/water mixtures, but cannot be dissolved in THF or dichloromethane, which show opposite solubility after the polymers are acidified with HCl, indicating reversibly tunable hydrophilicity of the polymers. Furthermore, water can participate in the MCP as the fourth component when the MCP is conducted in DMF with CuI as the catalyst and Na2CO3 as the base, generating random copolymers consisting of 3-alkenyloxindole moieties and two chiral center-containing 3-hydroxyindole moieties in the backbone. Some of the oxindole-containing poly(N-acylsulfonamide)s exhibit yellow to red emission in their solid state. These MCPs provide an efficient approach for the synthesis of functional polymers with unique structures, which directly build the oxindole and N-acylsulfonamide moieties in situ, demonstrating high synthetic efficiency.
- This article is part of the themed collection: Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...