Lipase-catalyzed synthesis of chiral poly(ester amide)s with an alternating sequence of hydroxy acid and l/d-aspartate units†
Abstract
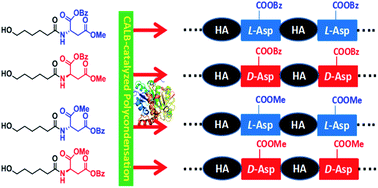
Most of reported synthesis approaches for poly(ester amide)s suffer from the difficulty in controlling the sequence of amino acid and ester units, and also some environmental disadvantages. Herein, we developed a facile approach for the lipase-catalyzed synthesis of chiral L-/D-Asp-based poly(ester amide)s with alternating aspartate and hydroxyhexanoic acid (HA) units. In the Novozym 435-catalyzed polycondensation starting from four monomers, namely N-(6-hydroxyhexanoyl) aspartate diesters with an L- or D-aspartate configuration, and methyl or benzyl ester groups at the α or β-carbonyl positions of Asp, we successfully obtained the corresponding alternating poly(HA-alt-L/D-β-aspartate)s with α-benzyl or α-methyl ester groups. The L-aspartate diester monomer was preferred to the D-aspartate diester monomer in the Novozym 435-catalyzed polycondensation, and it also showed high regioselectivity, mainly forming the β-linkage of the aspartate unit. Among the four poly(ester amide)s, poly(HA-alt-L-α-benzyl-β-aspartate) has the highest molar mass of up to 13 000 Da and 98% β-linkage. The thermal properties and the aggregate microstructures of these alternating poly(HA-alt-L/D-aspartate)s were also compared and some interesting differences were observed. Our results also showed that these enantiopure poly(HA-alt-L/D-aspartate)s did not form a stereocomplex.


 Please wait while we load your content...
Please wait while we load your content...