A new echelon of precision polypentenamers: highly isotactic branching on every five carbons†
Abstract
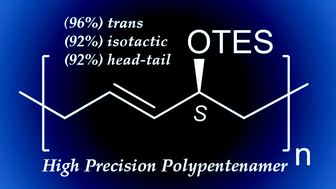
A series of allylic substituted cyclopentene monomers with increasing steric bulk are investigated by ring opening metathesis polymerization to understand the effects of these substituents on the microstructural outcomes of the resulting polypentenamers. 3-Triethylsiloxycyclopentene is discovered to polymerize to a highly regular microstructure with up to 96% trans olefins and 92% head-to-tail positional isomers. Dynamic resolution after synthetic modifications produced an enantiopure version (∼92% ee) of this monomer which subsequently produced a precision isotactic and regioregular polypentenamer for the first time. Synthetic investigations, mechanistic aspects, and basic thermal properties are discussed.
- This article is part of the themed collection: Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...