A supramolecular peptide polymer from hydrogen-bond and coordination-driven self-assembly†
Abstract
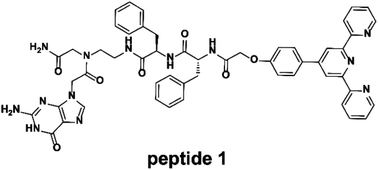
A terpyridine- and guanine-functionalized peptide was developed that could form different morphologies by self-assembly or coordination with Fe2+ in dimethyl sulfoxide. The self-assembly of the peptide is attributed to the G-quartet formation of a guanine moiety and intermolecular terpyridine π–π stacking. Upon the addition of Fe2+, a Fe2+–(terpyridine)2 complex is formed that turns the square-planar self-assembly to a three-dimensional self-assembly. As a consequence, a variety of interesting morphologies and chemical properties were observed. The self-assembled polymers were studied by nuclear magnetic resonance spectroscopy, ultraviolet-visible and fluorescence spectroscopy, viscosity measurement, circular dichroism, Fourier transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy, transmission electron microscopy, dynamic light scattering, and atomic force microscopy. This external stimuli driven self-assembly of a peptide may be further applied to drug delivery applications.


 Please wait while we load your content...
Please wait while we load your content...