Surface-attached poly(phosphoester)-hydrogels with benzophenone groups†
Abstract
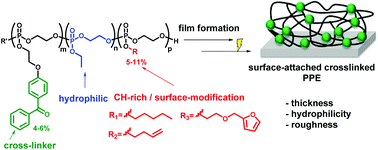
Photo-reactive poly(phosphoester)s (PPEs) forming surface-attached PPE-networks and hydrogels are presented. These contain benzophenone groups, which can photochemically cross-link polymer films via a C,H-insertion cross-linking (CHiC) reaction. To make the target polymers, a benzophenone-functionalized cyclic phosphate monomer was synthesized and copolymerized with ethylene ethyl phosphate (EEP). Polymerization with EEP and further cyclic phosphates gave terpolymers with additional chemical functionality (e.g. furfuryl or alkene groups). The resulting PPE copolymers and terpolymers were water-soluble and UV-reactive. Copolymerization kinetics of up to three different comonomers were studied by real-time 31P NMR, indicating a gradient-like structure of the copolymers and terpolymers. Cross-linked, surface-attached PPE-networks were formed by spin-coating these polymers onto pre-functionalized substrates, followed by UV irradiation. The resulting surface-attached polymer networks were smooth and hydrophilic (static contact angles of 20–26°). Since PPEs are biocompatible, these networks might be potential anti-fouling coatings for biomedical devices as implants or catheters. The furan and alkenyl groups in two of the terpolymers present a selectively addressable chemical function to further tune the surface properties of PPE-films.


 Please wait while we load your content...
Please wait while we load your content...