Photocatalysis and self-catalyzed photobleaching with covalently-linked chromophore-quencher conjugates built around BOPHY†
Abstract
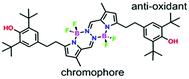
Two Chromophore-Quencher Conjugates (CQCs) have been synthesized by covalent attachment of the anti-oxidant dibutylated-hydroxytoluene (BHT) to a pyrrole-BF2 chromophore (BOPHY) in an effort to protect the latter against photofading. In fluid solution, light-induced intramolecular charge transfer is favoured in polar solvents and helps to inhibit photo-bleaching of the chromophore. The rate of photo-fading, which scales with the number of BHT residues, is zero-order in polar solvents but shows a linear dependence on the number of absorbed photons. The zero-order rate constant shows an inverse correlation with the fluorescence quantum yield measured in the same solvent. Photo-bleaching in benzonitrile involves autocatalysis while reaction in cyclohexane shows an unexpected stoichiometry. NMR spectroscopy indicates initial damage takes place at the BHT unit and allows identification of a reactive hydroperoxide as being the primary product. In the presence of an adventitious substrate, this hydroperoxide is a photocatalyst for amide formation under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...