Colorimetric detection of iron and fluorescence detection of zinc and cadmium by a chemosensor containing a bio-friendly octopamine†
Abstract
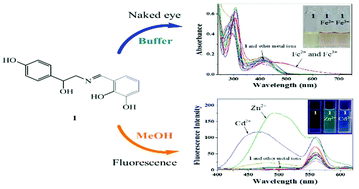
Chemosensor 1 was synthesized by the reaction of octopamine with 2,3-dihydroxybenzaldehyde. Sensor 1 determined iron with an obvious color change from pale yellow to brown in nearly-pure water. The detection limits (0.55 and 0.25 μM) for Fe2+ and Fe3+, respectively, were far lower than the EPA (Environmental Protection Agency) guideline concentration for drinking water (5.37 μM). In addition, 1 could be employed to quantify iron in environmental water samples. Moreover, sensor 1 exhibited different fluorescence emissions in response to zinc (green) and cadmium (blue). The binding structures and sensing mechanisms of 1 for iron, zinc and cadmium were proposed from various spectroscopic studies and theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...