A photochemical approach for evaluating the reactivity of substituted lappaconitines
Abstract
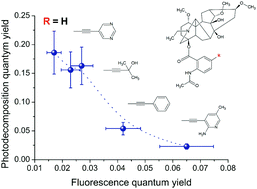
Lappaconitine (LC) is a natural diterpenoid alkaloid (DTA), acting as a human heart sodium channel blocker and possessing a wide range of biological activities, the cellular and molecular mechanisms of which are widely studied. This interest is due to the fact that various representatives of this DTA class show opposite biological activities. The possible reasons for this difference seem to be related to the peculiarities of the substituent effect on the drug–receptor binding process. In this work, the influence of substituents on the reactivity of LC and its derivatives has been studied by using elementary processes of photodecomposition. The given approach includes the joint analysis of the photophysical properties of the studied systems and their photodecomposition quantum yields. It allows us to trace the influence of substituents, located in the diterpenoid skeleton and anthranilic fragment, on processes in both moieties of LC. Summarizing the data obtained, an inverse dependence of fluorescence and photodegradation quantum yields has been observed. This correlation established for LCs, in particular, allows one to propose a way to evaluate the photostability of potential drugs based on fluorescence analysis. This would be appropriate for compounds in which the reactivity depends on intersystem crossing, i.e. in the cases where the initial and reacting excited states differ in multiplicity.


 Please wait while we load your content...
Please wait while we load your content...