Organogels composed of trifluoromethyl anthryl cyanostyrenes: enhanced emission and self-assembly†
Abstract
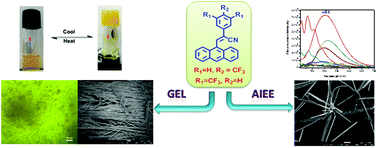
A series of α-cyanostyrenes bearing anthracene and electron withdrawing trifluoromethyl units were designed and synthesized. The α-cyanostyrene skeleton favors aggregation induced enhanced emission behavior due to the restriction of intramolecular rotations. Remarkably, the anthryl cyanostyrenes bearing simple trifluoromethyl (CF3) substituents form stable organogels with enhanced fluorescence emission compared to their solution state. In water, the CF3 substituted anthrylstyrenes self-assemble into entangled fibrous nano/microstructures through intermolecular H-bonding, π–π stacking and cyano substituent interactions. The morphological features of the aggregates and the gels were substantiated using scanning electron microscopy, TEM, and powder XRD measurements. The stability of the gels was assessed using rheology investigations.


 Please wait while we load your content...
Please wait while we load your content...