Synthesis and characterization of pyrrolyldipyrrin F-BODIPYs†
Abstract
A series of synthetic analogs of the tripyrrolic natural product prodigiosin were complexed with boron trifluoride to generate the corresponding F-BODIPYs. The maximum wavelengths of absorption and emission of the pyrrolyldipyrrin F-BODIPYs were tuned through variation of the substituents about the pyrrolyldipyrrinato core. The limited variation of substituents on the C-ring did not significantly affect absorption and emission. However, variation of substituents on the B-ring and A-ring resulted in a corresponding red-shift in absorption and emission reaching maximum wavelengths of 600 nm. The presence of electron donating substituents on the B-ring caused a decrease in the Stokes shift, while the presence of electron-withdrawing substituents caused an increase, ranging from 3–25 nm. Stokes shifts were solvent-dependant for some compounds. The inclusion of a dimethylamino group resulted in photo-induced electron transfer and thus quenched fluorescence which was restored upon protonation.
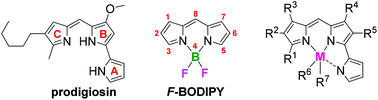


 Please wait while we load your content...
Please wait while we load your content...