Photoacidity as a tool to rationalize excited state intramolecular proton transfer reactivity in flavonols†
Abstract
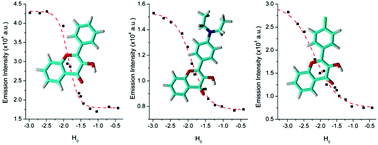
This work presents the determination of acidic strengths at the electronic ground and excited states (pKa and 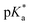 ) of three flavonol derivatives using electronic absorption and fluorescence emission spectroscopy. The differences of the pKa and
) of three flavonol derivatives using electronic absorption and fluorescence emission spectroscopy. The differences of the pKa and 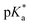 values were successfully correlated with the molecular structures according to the substitution pattern at the flavonol structure (hydrogen, diethylamino or fluoro moieties). In order to obtain more information about the observed photoacidity of these superacids, geometry optimizations and excitation energy calculations were performed at the CAM-B3LYP/6-311++G(d,p) level for their neutral, protonated and deprotonated species.
values were successfully correlated with the molecular structures according to the substitution pattern at the flavonol structure (hydrogen, diethylamino or fluoro moieties). In order to obtain more information about the observed photoacidity of these superacids, geometry optimizations and excitation energy calculations were performed at the CAM-B3LYP/6-311++G(d,p) level for their neutral, protonated and deprotonated species.


 Please wait while we load your content...
Please wait while we load your content...