Photochemical transformation of dimethyl phthalate (DMP) with N(iii)(H2ONO+/HONO/NO2−) in the atmospheric aqueous environment
Abstract
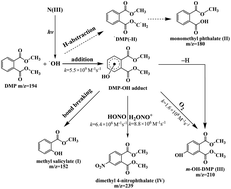
The photochemical transformation of dimethyl phthalate (DMP) with N(III)(NO2−/HONO/H2ONO+) was investigated using 365 nm steady-state irradiation and 355 nm laser flash photolysis (LFP) techniques. The results showed that N(III) concentration, DMP initial concentration and pH values all strongly affected the oxidation efficiency of DMP. The primary step of the reaction was the attack of ˙OH radicals on the aromatic ring to form a DMP–OH adduct, and the bimolecular rate constant was determined to be (5.5 ± 0.4) × 109 M−1 s−1. The DMP–OH adduct not only underwent monomolecular self-decay with a rate constant of (1.6 ± 0.3) × 104 s−1 but also interacted with HONO, H2ONO+ and O2 with rate constants of (6.4 ± 0.4) × 106 M−1 s−1, (8.8 ± 0.5) × 106 M−1 s−1 and (1.6 ± 0.1) × 108 M−1 s−1, respectively. Major transformation products including methyl salicylate, monomethyl phthalate, dimethyl 4-hydroxyphthalate and dimethyl 4-nitrophthalate were identified by GC-MS and characteristics of these secondary contaminants required extra attention.


 Please wait while we load your content...
Please wait while we load your content...