KHMDS mediated synthesis of 9-arylfluorenes from dibenzothiophene dioxides and arylacetonitriles by tandem SNAr-decyanation-based arylation†
Abstract
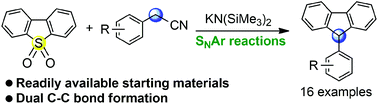
A straightforward KHMDS mediated synthetic route to 9-arylfluorenes from readily available starting materials has been developed. This reaction involves SNAr reactions of dioxide with arylacetonitriles, followed by decyanation reaction. The proposed transformation can also be used to furnish a densely arylated indene.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...