Competition between two cysteines in covalent binding of biliverdin to phytochrome domains†
Abstract
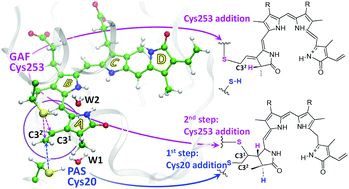
In this work, we disclose a mechanism of competing chemical reactions of protein assembly for a bacterial phytochrome using modern methods of molecular modeling. The recently designed variant of a near-infrared fluorescent protein miRFP670 shows novel and unexpected features of covalent binding of the biliverdin chromophore to cysteine residues in the phytochrome domains GAF and PAS. Upon protein assembly, biliverdin reacts either with a cysteine from GAF, or with two cysteines, one from GAF and one from PAS. We characterize computationally a model structure of the noncovalently bound biliverdin molecule inside the protein cleft of miRFP670 and model reactions of the covalent binding. Both cysteines, Cys20 (PAS) and Cys253 (GAF), are located close to the electrophile C32 atom of biliverdin and can act as nucleophiles. The nucleophilic attack of Cys253 from the GAF domain results in a single C–S bond formation with an activation energy of 16 kcal mol−1. Another pathway, leading to the biliverdin adduct with two C–S bonds, is characterized by lower energy barriers, less than 11 kcal mol−1. Competition between these reaction pathways explains the experimentally obtained mixture of both adducts. On the basis of our first simulations of covalent BV binding to the phytochrome domains, we propose an approach of a direct experimental validation of the reaction mechanisms using IR vibrational spectroscopy.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...