Preparation of polysubstituted dihydrofurans through a PhI(OAc)2-promoted haloenolcyclization of olefinic dicarbonyl compounds†
Abstract
A metal-free cyclization of olefinic dicarbonyl compounds for the synthesis of various 5-halomethyl-4,5-dihydrofurans is presented. Using (diacetoxyiodo)benzene as the reaction promoter and halotrimethylsilane as the halogen source, the intramolecular haloenolcyclization of the 2-allyl-1,3-dicarbonyl compounds smoothly proceeded, leading to the corresponding 5-halomethyl-4,5-dihydrofurans in good to excellent isolated yields. Moreover, the resulting 5-iodomethyl products could be converted to functionalized furans in almost quantitative yields by treatment with DBU followed by acid-catalyzed rearrangement. The reactions could be carried out on a gram scale and did not require harsh reaction conditions. The good isolated yields, mild conditions, and operational simplicity make this reaction a viable method for the construction of different dihydrofuran and furan structures.
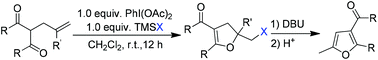
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...