Aminative Umpolung cyclization for synthesis of chiral exocyclic vicinal diamines†
Abstract
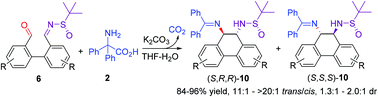
Chiral exocylic vicinal diamines are biologically and chemically important compounds, but they are not easy to make. In this paper, an interesting aminative Umpolung cyclization process has been developed. Aromatic aldehydes 6 bearing an electrophilic chiral sulfinimine group underwent imine formation with 2,2-diphenylglycine (2), decarboxylation, and subsequent Umpolung cyclization, producing various trans-diamines 10 in 84–96% yields with high trans/cis ratios under very mild conditions. This work not only provides an efficient, clean, and mild method for the synthesis of chiral exocyclic vicinal diamines in one step but also represents a new application of aminative Umpolung strategy on intramolecular reactions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...