Dirhodium(ii)/P(t-Bu)3 catalyzed tandem reaction of α,β-unsaturated aldehydes with arylboronic acids†
Abstract
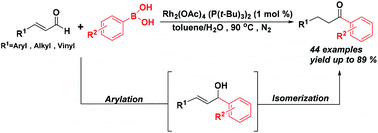
Phosphine ligated dirhodium(II) acetate is advocated as a catalyst for the synthesis of aryl alkyl ketones by the tandem reaction of α,β-unsaturated aromatic or aliphatic aldehydes with arylboronic acids. This tandem procedure included arylation followed by the isomerization reaction. This method exhibits good functional group tolerance and has a broad substrate scope. With the conjugated aldehydes, the one-step synthesis of γ,δ-unsaturated ketones was realized through this reaction. It is noteworthy that the length of the Rh–P bond is an important factor affecting catalytic reactions. The comparative analysis of the crystal structures of axially alkylphosphane and arylphosphane ligated dirhodium(II) acetate revealed that the shorter Rh–P bond length favors the isomerization process as compared to the longer one. In addition, the dirhodium(II) compound can be recovered after the completion of the reaction.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...