Palladium-catalyzed heck-type cascade cyclization of (Z)-1-iodo-1,6-dienes with N-tosyl hydrazones†
Abstract
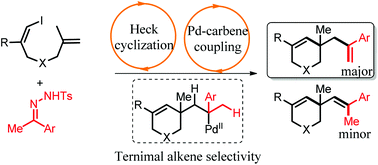
A palladium-catalyzed heck-type cascade cyclization of (Z)-1-iodo-1,6-dienes with N-tosyl hydrazones is reported. The alkylpalladium intermediate coupled with the diazo compound, generating the second alkylpalladium species bearing two β-H, which generated a terminal alkene as the major products in the anti-Zaitsev way via the highly regioselective β-H elimination. It provided a new way to synthesize tetrahydropyridine derivatives bearing a terminal alkene.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...