Silver(i)/base-promoted propargyl alcohol-controlled regio- or stereoselective synthesis of furan-3-carboxamides and (Z)-enaminones†
Abstract
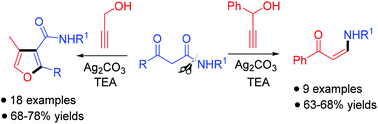
A novel and facile regioselective synthesis of furan-3-carboxamides by a silver(I)/base-promoted reaction of propargyl alcohol with 3-oxo amides has been demonstrated. This one-pot protocol provides a rapid synthetic approach to diverse trisubstituted furan-3-carboxamides via cascade nucleophilic addition, intramolecular cyclization, elimination, and isomerization reactions. Employing a substituted propargyl alcohol, (Z)-enaminones have been obtained with high stereoselectivities by a Ag2CO3-promoted reaction starting from 3-oxo amides via C–N bond cleavage.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...