A convenient stereoselective access to novel 1,2,4-triazepan-3-ones/thiones via reduction or reductive alkylation of 7-membered cyclic semicarbazones and thiosemicarbazones†
Abstract
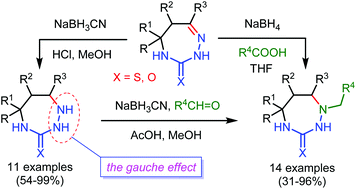
A general stereoselective approach to previously unknown 1,2,4-triazepane-3-thiones/ones based on reduction or reductive alkylation of readily available 2,4,5,6-tetrahydro-3H-1,2,4-triazepine-3-thiones/ones has been developed. The approach involved the treatment of tetrahydrotriazepines with sodium cyanoborohydride in MeOH at pH 3 or with sodium borohydride and excess of carboxylic acid in THF to give 1-unsubstituted or 1-alkyl-substituted 1,2,4-triazepane-3-thiones/ones, respectively. The latter were also prepared by the reaction of 1-unsubstituted 1,2,4-triazepane-3-thiones/ones with sodium cyanoborohydride and an aldehyde in MeOH in the presence of AcOH. The stereochemistry of the triazepane-3-thiones/ones obtained was established in DMSO solution and in the solid state using NMR spectroscopy and single-crystal X-ray diffraction. These data and DFT B3LYP/6-311++G(d,p) calculations show that the gauche effect plays a significant role in stabilization of preferred conformations of 1-unsubstituted 1,2,4-triazepane-3-thiones/ones.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...