Diisopropylethylamine-triggered, highly efficient, self-catalyzed regioselective acylation of carbohydrates and diols†
Abstract
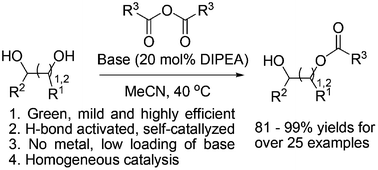
A diisopropylethylamine (DIPEA)-triggered, self-catalyzed, regioselective acylation of carbohydrates and diols is presented. The hydroxyl groups can be acylated by the corresponding anhydride in MeCN in the presence of a catalytic amount of DIPEA. This method is comparatively green and mild as it uses less organic base compared with other selective acylation methods. Mechanistic studies indicate that DIPEA reacts with the anhydride to form a carboxylate ion, and then the carboxylate ion could catalyze the selective acylation through a dual H-bonding interaction.


 Please wait while we load your content...
Please wait while we load your content...