Sterically shielded tetrazoles for a fluorogenic photoclick reaction: tuning cycloaddition rate and product fluorescence†
Abstract
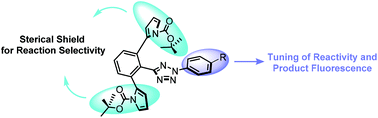
A panel of sterically shielded tetrazoles with different N-aryl groups were synthesized and subsequently evaluated in the photoinduced tetrazole–alkene cycloaddition reaction. It was found that increase in the HOMO energy of the corresponding nitrile imines leads to a faster cycloaddition reaction along with a red shift in the fluorescence emission of the pyrazoline cycloadduct.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...