Electrophilic carbocyclization reactions of 2-(2-alkynylphenyl)amino-1,4-naphthoquinones†
Abstract
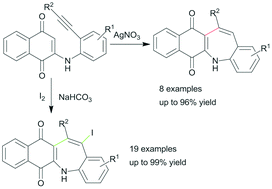
An efficient Ag(I)-catalyzed method for the synthesis of 5H-benzo[b]naphtho[2,3-f]azepine-6,11-diones from 2-(2-ethynylphenyl)amino substituted 1,4-naphthoquinones has been developed. In this transformation new C–C bond formation occurred regioselectively via a 7-endo-dig cyclization. The related 13-iodine substituted benzazepine derivatives could also be produced exclusively by the I2-induced carbocyclization reaction of 2-(2-alkynylphenyl)amino substituted 1,4-naphthoquinones. This process has advantages such as mild reaction conditions and tolerance of a broad range of functional groups.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...