Unprecedented synthesis of 1,2,3-triazolo-cinnolinone via Sonogashira coupling and intramolecular cyclization†
Abstract
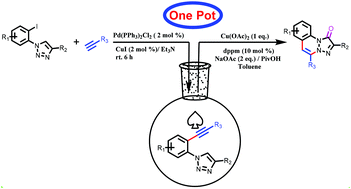
An unprecedented copper mediated one-pot sequential synthesis of 1,2,3-triazolo cinnolinone derivatives from 2-halo-phenyl triazoles and terminal alkynes has been reported. Under the optimized reaction conditions, a broad range of substituted triazoles and alkynes were found to participate in this transformation, thus affording unknown 1,2,3-triazolo cinnolinone derivatives in moderate to excellent yields. This method proceeds through sequential C–C coupling followed by an annulation cascade sequence in the same vessel under atmospheric air as the sole oxidant, thus representing a simple, efficient and atom economical approach for the synthesis of aza-cinnolinones.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...