Synthesis of substituted piperidines by enantioselective desymmetrizing intramolecular aza-Michael reactions†
Abstract
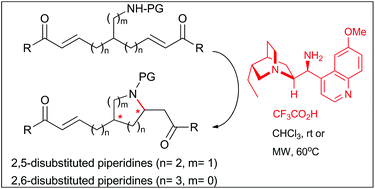
An organocatalytic enantioselective intramolecular aza-Michael reaction has been described for the first time in a desymmetrization process employing substrates different from cyclohexadienones. By using 9-amino-9-deoxy-epi-hydroquinine as the catalyst and trifluoroacetic acid as a co-catalyst, a series of enantiomerically enriched 2,5-and 2,6-disubstituted piperidines have been obtained in good yields and with moderate diastereoselectivity. Depending on the catalyst/co-catalyst loading ratio, either the major or the minor diastereoisomer of the final piperidine products was achieved with high levels of enantioselectivity. Finally, some mechanistic insights have been considered by means of theoretical calculations which were in agreement with the experimental results obtained in the desymmetrization reaction.


 Please wait while we load your content...
Please wait while we load your content...