A new route to substituted furocoumarins via copper-catalyzed cyclization between 4-hydroxycoumarins and ketoximes†
Abstract
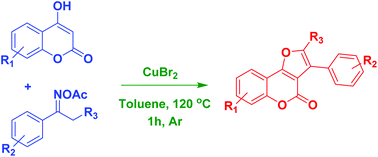
A new route to substituted furocoumarins via copper-catalyzed cyclization between 4-hydroxycoumarins and ketoximes was developed. CuBr2 exhibited higher activity than other copper salts, affording the desired furocoumarins in high yields. The transformation proceeded readily in the absence of stoichiometric external oxidants. The significance of this synthetic strategy would be (1) the easily available starting materials; (2) low cost catalyst CuBr2; and (3) being without stoichiometric external oxidants. This protocol is complementary to previous approaches in the synthesis of substituted furocoumarins.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...