A convergent strategy towards febrifugine and related compounds†
Abstract
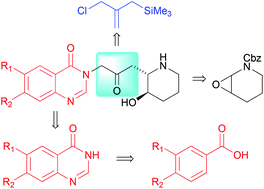
We report a modular five step synthetic route to the febrifugines that employs 2-(chloromethyl)allyl-trimethylsilane as a conjunctive reagent for the coupling of the piperidine and quinazolinone groups. We also demonstrate the application of a recent Rh-catalyzed quinazolinone synthesis for the facile generation of febrifugine analogs.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...