Phosphine-catalysed α-umpolung addition of nucleophiles to δ-acetoxy allenoates: stereoselective synthesis of 2,4-dienoates†
Abstract
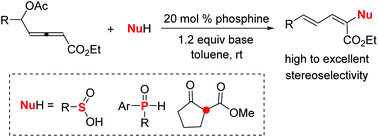
The first example of phosphine-catalyzed α-umpolung addition of nucleophiles to allenoates is described, which features the use of δ-acetoxy allenoate to generate a 3-phosphonium-2,4-dienoate intermediate, thus facilitating the α-umpolung addition of nucleophiles. Both sulfinate and diarylphosphine oxide are competitive nucleophiles, affording highly activated conjugated dienes with good to excellent stereoselectivities.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...