Chelation-assisted C–N cross-coupling of phosphinamides and aryl boronic acids with copper powder at room temperature†
Abstract
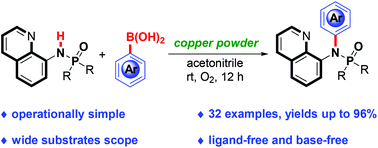
A protocol for the chelation-assisted C–N cross-coupling of phosphinamides and aryl boronic acids with copper powder under an oxygen atmosphere is reported. This reaction proceeds efficiently to afford fully substituted unsymmetrical N-arylation phosphinamides at room temperature in excellent yields. Diverse unstable functional groups on the benzene ring of aryl boronic acids such as vinyl, formyl, acetyl, sulfonyl, acetylamino, cyano, nitro, and trifluoromethyl can be accommodated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...