Synthesis of multi-substituted allenes from organoalane reagents and propargyl esters by using a nickel catalyst†
Abstract
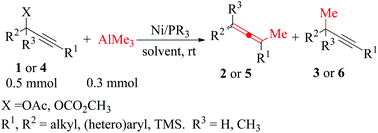
A highly efficient and simple route for the synthesis of multi-substituted allenes has been developed by a nickel catalyzed SN2′ substitution reaction of propargyl esters with organic aluminium reagents under mild conditions, which gave the corresponding multi-substituted allenes in good to excellent yields (up to 92%) and high selectivities (up to 99%) at 60 °C for 6 h in THF. Aryls bearing electron-donating or electron-withdrawing groups in propargyl esters gave products in good yields. In addition, the multi-substituted allenes bearing a thienyl or a pyridyl group were obtained in 95–97% selectivities with isolated yields of 72–83%. Furthermore, the SN2′ substitution reaction worked efficiently with propargyl carbonate compounds as well. On the basis of the experimental results, a possible catalytic cycle has been proposed.


 Please wait while we load your content...
Please wait while we load your content...