Electronic effect of substituents on anilines favors 1,4-addition to trans-β-nitrostyrenes: access to N-substituted 3-arylindoles and 3-arylindoles†‡
Abstract
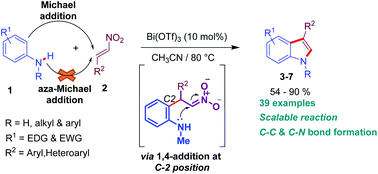
A simple and an efficient method for the regioselective synthesis of N-alkyl/aryl/H 3-arylindole derivatives from N-substituted anilines and trans-β-nitrostyrenes has been described using 10 mol% of bismuth(III) triflate as a catalyst in acetonitrile at 80 °C. The present protocol profits from the formation of new C–C and C–N bonds, broad substrate scope and moderate to good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...