One-pot synthesis of 1,5-diketones from 3-acetyl-4-hydroxycoumarin and effective cyclization to unexpected 3,4-dihydropyridines†
Abstract
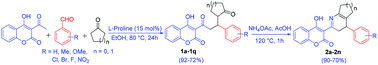
A facile synthesis of 1,5-diketones from 3-acetyl-4-hydroxycoumarin, aldehydes and cyclic-ketones via a one-pot aldol condensation and subsequent Michael addition reaction in the presence of a single catalyst of L-proline under mild reaction conditions has been developed. Novel 1,5-diketones were further cyclized to unexpected 3,4-dihydropyridines rather than generally formed pyridine analogues with ammonium acetate in acetic acid. One pot, high yields (72–92%) for novel 1,5-diketones and (70–90%) for the 3,4-dihydropyridine adducts, easy work-up and purification of products are the key advantages of this method.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...