Facile synthesis of 2,3-benzodiazepines using one-pot two-step phosphate-assisted acylation–hydrazine cyclization reactions†
Abstract
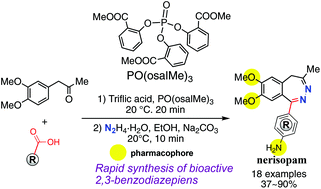
Here, we report new methodology for synthesizing 2,3-benzodiazepines and their analogues by means of phosphate-assisted acylation reaction of 1-arylpropan-2-ones with a carboxylic acid followed by hydrazine cyclization in a one-pot two-step manner. An unprotected amino group is tolerated in this reaction. This method provides a direct access to 2,3-benzodiazepines containing aromatic 7,8-dimethoxy and 1-p-aminophenyl groups, which are generally considered important for bioactivity. The presence of 3,4-dimethoxy or 3-methoxy substitution on the benzene ring of the 1-arylpropan-2-one is important for high regioselectivity in the acylation reaction.


 Please wait while we load your content...
Please wait while we load your content...