An organocatalytic asymmetric Mannich reaction of pyrazoleamides with cyclic trifluoromethyl ketimines: enantioselective access to dihydroquinazolinone skeletons†
Abstract
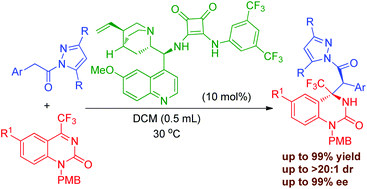
An organocatalyzed asymmetric Mannich reaction of pyrazoleamides and cyclic trifluoromethyl ketimines with a chiral bifunctional amine-squaramide as the catalyst was developed. A wide range of trifluoromethyl dihydroquinazolinone derivatives bearing adjacent quaternary and tertiary stereogenic centers were readily obtained in good to excellent yields (up to 99%) with high diastereo- and enantioselectivities (up to 99% ee and >20 : 1 dr). The large scale experiment and transformation of the product have also been demonstrated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...