NFSI-participated intermolecular aminoazidation of alkene through iron catalysis†
Abstract
An iron-catalysed intermolecular vicinal aminoazidation of alkenes, using N-fluorobenzenesulfonimide (NFSI) and trimethylsilyl azide (TMSN3) as the imidating and azidating reagents, respectively, is described, which could potentially provide a valuable route toward diverse vicinal diamine derivatives of great significance in medicinal chemistry and organic synthesis. Such iron-catalysed aminative bisfunctionalization of alkenes with NFSI has not been reported yet. Comparing to previously employed copper or palladium catalysts, the iron catalyst, FeCl2, was demonstrated to be a good alternative for its comparable efficiency and broad alkene scope. Preliminary mechanistic study suggested that this iron-catalysed reaction is realized through radical processes.
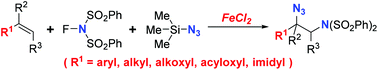
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...