Design and synthesis of novel monoterpenoid indole alkaloid-like analogues and their antitumour activities in vitro†
Abstract
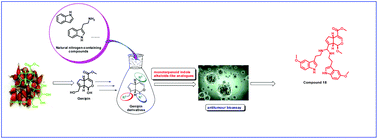
A biomimetic synthetic strategy and combinatorial chemistry were used to synthesize 34 novel monoterpenoid indole alkaloid (MIA) analogues, and their cytotoxic activities against five cancer cell lines (SW-480, A-549, HL-60, SMMC-7721, and MCF-7) were determined using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Fourteen of these analogues (7, 16–18, and 23–32) showed significantly greater inhibition of tumour cell proliferation than cisplatin. Compounds 17 and 18 showed the highest cytotoxic activity against the HL-60 cell line with IC50 values of 0.90 μM and 0.43 μM, respectively. Compound 18 slightly induced apoptosis and arrested the cell cycle in SW-480, A-549, HL-60, SMMC-7721, and MCF-7 cells. Analysis of the primary structure–activity relationships reveals that the introduction of different substituent groups at the C-3, C-5, and C-6 positions of the indole moiety and the C-10 position of the genipin moiety might have an effect on the antitumour activity of the resulting compounds.


 Please wait while we load your content...
Please wait while we load your content...