2,3-Diaroyl benzofurans from arynes: sequential synthesis of 2-aroyl benzofurans followed by benzoylation†
Abstract
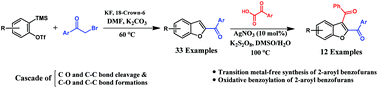
A cascade synthetic strategy for the direct synthesis of 2-aroyl benzofurans from aryne precursors has been developed. This reaction proceeds via C–O and C–C bond cleavage as well as C–O and C–C bond formation in a single reaction vessel. The methodology provides good yields of 2-aroyl benzofurans and tolerates a variety of functional groups. The synthesized 2-aroyl benzofurans were further benzoylated at 3-positions and one of the synthesized 2,3-diaroyl benzofuran structures was confirmed unambiguously by X-ray crystallography.


 Please wait while we load your content...
Please wait while we load your content...