Formal synthesis of cis-solamin: acid-catalyzed one-step construction of 2,5-disubstituted tetrahydrofuran†
Abstract
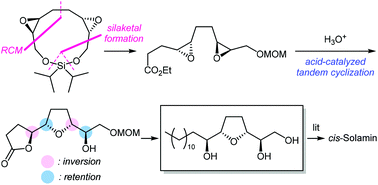
A divergent strategy has been used for the concise and efficient enantioselective formal synthesis of Annonaceous acetogenin cis-solamin. Our synthetic strategy comprises concise preparation of the diepoxyester via an 11-membered silaketal constructed by ring-closing metathesis after the dimerization of chiral epoxides, and uses an acid-catalyzed tandem intramolecular SN2-like reaction to construct the threo-cis-threo configuration of the tetrahydrofuran-diol moiety.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...