Enantioselective amination of nitroolefins under base-free and water-rich conditions using chiral bifunctional phase-transfer catalysts†
Abstract
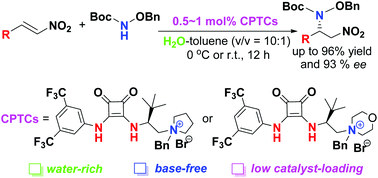
The direct enantioselective amination of nitroolefins has been performed with L-tert-leucine-derived squaramide-scaffold bifunctional phase-transfer catalysts under base-free and water-rich conditions with low catalyst loading (0.5–1 mol%) to provide 2-aminonitroalkanes in good yields (up to 96%) and enantioselectivities (up to 93% ee).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...