Facile access to 2-acyloxy-, aryloxy- and alkenyloxy-2H-azirines via an SN2′–SN2′ cascade in 2-halo-2H-azirines†
Abstract
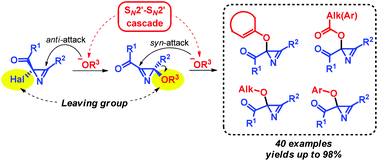
Various 2-oxygen-substituted 2H-azirine-2-carboxylic acid derivatives were synthesized in high yields under mild conditions from readily available precursors, 2-halo-2H-azirines and OH-reagents having pKa values in the range of 3–10. This reaction is the first example of substitution at the azirine carbon atom for which an unusual SN2′–SN2′ cascade mechanism was revealed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...