Neighboring phenolic group-activated gem-difluoroallylboration of imines for the catalyst-free synthesis of gem-difluorohomoallylamines†
Abstract
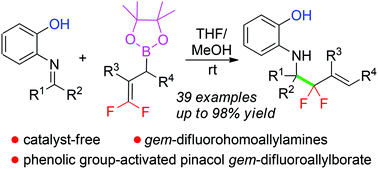
We herein report an unprecedented addition reaction of pinacol gem-difluoroallylborates and imines enabled by a neighboring phenolic group in an N-protecting group under catalyst-free conditions, thus facilitating the construction of a wide range of racemic gem-difluorohomoallylamine derivatives. Based on the control experiments, a plausible transition state via the Zimmerman–Traxler model was proposed to elucidate the significance of the neighboring phenolic group, as well as the γ-selectivity of the boronate reagents.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...