Ruthenium(ii)-catalyzed remote C–H addition of 8 aminoquinoline amide to activated aldehyde†
Abstract
Ruthenium(II)-catalyzed regioselective remote C–H addition of 8-aminoquinoline amides at C-5 position to ethyl glyoxalate and 2,2,2-trifluoroacetaldehyde have been developed. The transformation affords C-5 functionalized quinolines in moderate to good yields. This method is applicable to various aryl, heteroaryl as well as aliphatic carboxamides. Experimental results suggest that the reaction very likely proceeds through an ionic pathway.
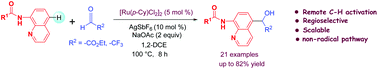
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...