Stereoselective preparation of key intermediates for the synthesis of iso-, neuro- and phyto-prostane family members: inaugural asymmetric synthesis of 17-E2c-dihomo- and 17-F2c-dihomo-isoprostanes†
Abstract
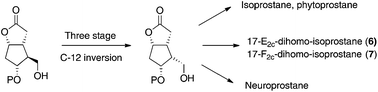
A practical methodology for the synthesis of key intermediates for isoprostane, neuroprostane and dihomo-isoprostane preparation has been described. The key strategy involved a three stage C-12 stereocenter inversion of the configuration of a Corey lactone, commercially available in an enantiopure form. The key intermediate was then used to prepare 17-E2c-dihomo-isoprostane and 17-F2c-dihomo-isoprostane.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...