Palladium-catalyzed carbonylation of benzylic ammonium salts to amides and esters via C–N bond activation†
Abstract
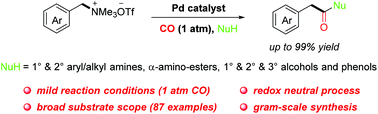
An efficient palladium-catalyzed carbonylation reaction of readily available quaternary ammonium salts with CO is reported for the first time to afford arylacetamides and arylacetic acid esters via benzylic C–N bond cleavage. This protocol features mild reaction conditions under atmospheric pressure of CO, a redox-neutral process without an additional oxidant, and a broad substrate scope for various kinds of amines, alcohols and phenols.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...